Chemistry
Pure gold precipitate produced by the aqua regia refining process
Although gold is a noble metal, it forms many and diverse compounds. The oxidation state of gold in its compounds ranges from −1 to +5, but Au(I) and Au(III) dominate its chemistry. Au(I), referred to as the aurous ion, is the most common oxidation state with soft ligands such as thioethers, thiolates, and tertiary phosphines. Au(I) compounds are typically linear. A good example is Au(CN)2−, which is the soluble form of gold encountered in mining. Curiously, aurous complexes of water are rare. The binary gold halides, such as AuCl, form zigzag polymeric chains, again featuring linear coordination at Au. Most drugs based on gold are Au(I) derivatives.[56]
Au(III) (auric) is a common oxidation state and is illustrated by gold(III) chloride, AuCl3. Au(III) complexes, like other d8 compounds, are typically square planar.
Aqua regia, a 1:3 mixture of nitric acid and hydrochloric acid, dissolves gold. Nitric acid oxidizes the metal to +3 ions, but only in minute amounts, typically undetectable in the pure acid because of the chemical equilibrium of the reaction. However, the ions are removed from the equilibrium by hydrochloric acid, forming AuCl4− ions, or chloroauric acid, thereby enabling further oxidation.
Some free halogens react with gold.[57] Gold also reacts in alkaline solutions of potassium cyanide. With mercury, it forms an amalgam.
Less common oxidation states
Less common oxidation states of gold include −1, +2, and +5.
The −1 oxidation state occurs in compounds containing the Au− anion, called aurides. Caesium auride (CsAu), for example, crystallizes in the caesium chloride motif.[58] Other aurides include those of Rb+, K+, and tetramethylammonium (CH3)4N+.[59]
Gold(II) compounds are usually diamagnetic with Au–Au bonds such as [Au(CH2)2P(C6H5)2]2Cl2. The evaporation of a solution of Au(OH)3 in concentrated H2SO4 produces red crystals of gold(II) sulfate, AuSO4. Originally thought to be a mixed-valence compound, it has been shown to contain Au4+2 cations.[60][61] A noteworthy, legitimate gold(II) complex is the tetraxenonogold(II) cation, which contains xenon as a ligand, found in [AuXe4](Sb2F11)2.[62]
Gold pentafluoride and its derivative anion, AuF−6, is the sole example of gold(V), the highest verified oxidation state.[63]
Some gold compounds exhibit aurophilic bonding, which describes the tendency of gold ions to interact at distances that are too long to be a conventional Au–Au bond but shorter that van der Waals bonding. The interaction is estimated to be comparable in strength to that of a hydrogen bond
Mixed valence compounds
Well-defined cluster compounds are numerous.[59] In such cases, gold has a fractional oxidation state. A representative example is the octahedral species {Au(P(C6H5)3)}62+. Gold chalcogenides, such as gold sulfide, feature equal amounts of Au(I) and Au(III).
We deals in precious jewelry made up of Diamond and Gold. Sale purchase of Jewelry / new online deals / home delivery and many more information about jewelry
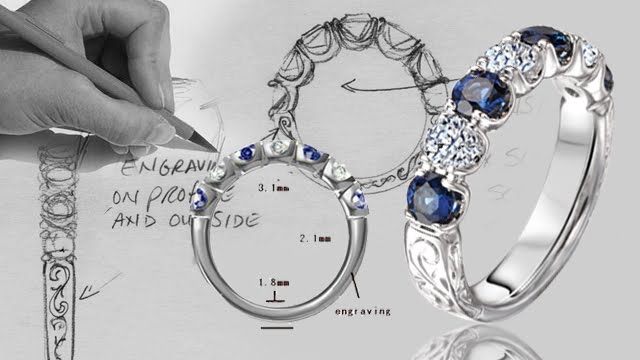
Thursday, June 3, 2010
Subscribe to:
Post Comments (Atom)
5 NEW YEAR GIFTS FOR YOUR GIRL FRIEND UNDER THE BUDGET OF 50$
GIRLS WOULD LOVE IT. Don’t waste the time and enjoy your golden moments with your loved once. Let’s celebrate the 2021 with loved once...

Online order delivery form / contact us
-
Diamond Rings are available for sale on very reasonable price. 05% discount limited offer contact now. For furthur detail. E-mail u...
-
ALL THESE BANGLES ARE AVAILABLE FOR SALE, CONTACT DETAIL EMAIL US: r.alirashid@yahoo.com CALL US: +9...
-
Number 1 HXZZ Fine Jewelry Women Gifts 925 Sterling Silver Freshwater Cultured Teardrop White Pearl Pendant Necklace Customer ...




No comments:
Post a Comment